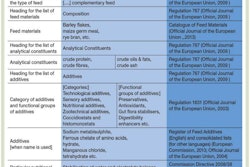
The U.S. Food and Drug Administration has announced that five drug sponsors holding animal drug applications affected by Guidance For Industry (GFI) #213 have requested that FDA withdraw approval of a collective 19 animal drug applications because the products are no longer manufactured or marketed. Of these 19 applications, 16 are antimicrobials affected by GFI #213.
The guidance outlines FDA’s plan to help curb antimicrobial resistance by, among other things, phasing out the use of medically important antimicrobials in food-producing animals for production purposes.
The following companies have requested that FDA withdraw approval for their listed products:
ADM Alliance Nutrition Inc.
- Gilt Edge TYLAN (tylosin phosphate) Mix
- HFA Tylosin-10 Plus Sulfa (tylosin phosphate and sulfamethazine)
- Good Life TYLAN 10 (tylosin phosphate) Premix
- HFA HYGROMIX 0.48 (hygromycin B) Medicated Premix
- TYLAN 5 Sulfa (tylosin phosphate and sulfamethazine) Premix
Micro Beef Technologies LTD
- TYLAN 40 or 100 (tylosin phosphate)
Ridley Feed Ingredients
- Waynextra for Swine (tylosin phosphate)
- TYLAN Sulfa-G (tylosin phosphate and sulfamethazine)
- Ban-A-Worm II (pyrantel tartrate)
Provimi North America Inc.
- TYLAN 5, 10, 20, or 40 (tylosin phosphate)
- WORM-BAN 5 or 10 (pyrantel tartrate)
- HYGROMIX 0.6 (hygromycin B)
- FLAVOMYCIN 0.4 or 2 (bambermycins)
- STAFAC 10 (virginiamycin)
Virbac AH Inc.
- PURINA HYGROMIX (hygromycin B) for Swine
- PURINA Pork-Plus (tylosin phosphate and sulfamethazine)
- PURINA Hog Plus II (tylosin phosphate)
- PURINA TYLAN 40 (tylosin) Plus Sulfamethazine
- PURINA Check-R-Ton Ll. (lincomycin hydrochloride)