While the impact of the new European regulations on what must be declared on a compound feed label is easy to see, it may not be so obvious that their approval has also led to the establishment of a specific “compliant” terminology to be used in some of the label sections. Using other terms will result in “wrong” labeling and may be regarded as a non-compliance by European Feed Manufacturers’ Federation (FEFAC), which can have consequences in terms of time (negotiation with national authorities) and money (fines). It is, thus, not a minor issue, but rather an important consideration when preparing a label.
All EU languages need to be considered
The importance of using this specific European terminology increases dramatically when the compound feed is to be marketed in multiple EU countries. Indeed, according to the Official Journal of the European Union, the label needs to be translated into at least one of the official languages of each country in which the product will be placed. As the regulations are available in all of the official EU languages, it goes without saying that the “regulated” label terms will need to be used not only on the English label, but also the French, Spanish, German, Dutch, Italian, Czech, Finnish, Danish and Greek labels, to name just a few.
Clearly, this can pose a challenge when a company is targeting the entire EU, as up 23 languages will need to be considered. The more languages, the greater the risk of getting one of the regulated terms wrong. The situation is especially delicate when there is no suitable process in place to ensure that a label is translated in line with the compliant wording specified in each regulation.
Categories and ingredient labels
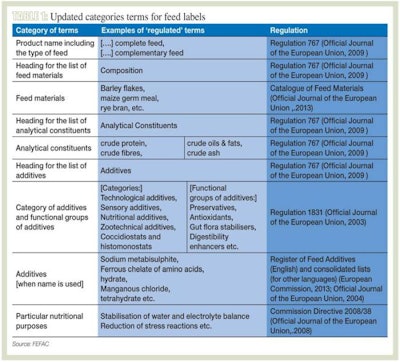
When drafting an English label and choosing the resources for its translation, it is therefore crucial to establish a process or rely on a partner able to identify the main categories of terms affected by the legislation and the applicable regulations in each case. As a general guide, Table 1 lists these categories – along with example terms – and shows which regulation should be used to pick the right term.
One of the most striking features of the categories included in Table 1 is the sheer variety of terms involved. Not only the headings and the wording to be used for the categories of additives declared, but also the names of the analytical constituents and the terms to be used for the feed materials and the intended uses of PARNUTs (or “Foods For Particular Nutritional Use”) are all officially established and described in the different regulations and directives. It is thus no longer a question of finding the proverbial needle in the haystack, but of sifting through and deciphering each piece of hay.
For feed ingredients, it is worth noting that while use of the Catalog of Feed Materials is voluntary, i.e. other terms may be used on the label provided they properly describe the feed materials used, the terms included in it may only be used for ingredients that match the descriptions it provides. This may seem an easy-enough task when only one language is involved, but it can be laborious and time-intensive when all 23 language versions of the Catalog must be reviewed. Again, a suitable process or specialized partner must be selected to ensure that the right feed material names are used when needed.
Separate labels for non-EU markets
Some may also be surprised to notice that Europe has shifted from the previously widespread “Ingredients” section to the newly accepted “Composition” heading. Similarly, the former “Guaranteed Analysis” is no longer a valid heading; “Analytical Constituents” should be used instead. In this sense, labels following the Association of American Feed Control Officials’ (AAFCO) recommendations (where “Ingredients” and “Guaranteed Analysis” are still the preferred terms) differ from EU-compliant labels. For non-EU markets that share one of the languages used in the EU, such as Spanish-speaking Latin America and Spain, the text on the label will need to reflect both regulatory realities. Otherwise, a separate label will need to be prepared for each market.
Preparing the right packaging, including all the compliant labels required for EU target markets, can be a complex task for compound feed producers. The new regulations have established not only a different legislative framework, but also a whole set of specific terminology that must be meticulously applied in order to avoid time-consuming negotiations and potential fines. Putting into place a reliable system for packaging design and translation for products to be exported elsewhere in Europe and abroad is now essential. In short, when it comes to feed, words matter.















