Bacteriophages reproduce by utilizing the host cell’s machinery to replicate viral proteins and genomic material — this kills the bacteria in the process. Understanding the molecular processes of their life cycle, particularly the cell lysis steps, has led to the isolation of viral proteins. With the continued drive to reduce antibiotic use, bacteriolytic agents could be exploited therapeutically as antibacterial agents.
Bacteriophages are viruses that infect and kill bacteria using the bacterium’s own metabolisms to produce new virus particles and have the potential to be used as alternatives to antibiotics. With few new antibiotics in development and the threat of resistance mounting, progressing these innovative concepts into animal and human trials should be a priority.
Research into the identification, production and use of phage lytic enzymes suggests bacteriophages can be used as antibacterial agents against Clostridia perfringens, a pathogen that harms humans and poultry.
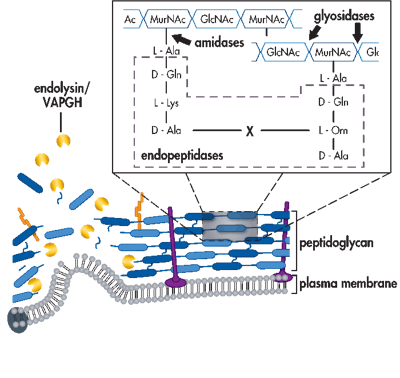
Schematic of bacterial cell envelope attacked by phage-encoded proteins during lytic replication. Endolysins and holins work in concert for cell lysis during the final stages of replication. Virion-associated peptidoglycan hydrolases are used by certain phages to puncture through the bacterial cell envelope. Depolymerases degrade the extracellular polysaccharides and lipopolysaccharides that can be barriers to viral cell surface adsorption. (Roach & Donovan, 2015)
Phage lytic enzymes
Lytic phages split bacterial cells open and destroy them, enabling the new phage to infect another host. Their specificity against specific bacterial species, without negatively effecting beneficial bacteria within the gut, make them attractive alternatives to antibiotics.
Because phage receptors/anti-receptors evolve very quickly, phages are rendered less likely to quickly kill their hosts; however, utilization of mechanisms such as bacteriophage lysins to target bacterial pathogens are found to be a superior method, says Bruce Seal, former research leader for the Poultry Microbiological Safety Research Unit of the U.S. Department of Agriculture’s (USDA) Agricultural Research Service (ARS) and retired biology professor at the Oregon State University – Cascades.
Phage endolysins are cell wall hydrolases that are produced to help the new phage escape the infected host. Depolymerases assist the viral particle in adsorbing, invading and disintegrating the host bacteria, an activity that can continue even within biofilms. Holins are responsible for disruption of the cytoplasmic membrane to assist endolysins in cell lysis.
This article will focus on the application of endolysins against Gram-positive bacteria.
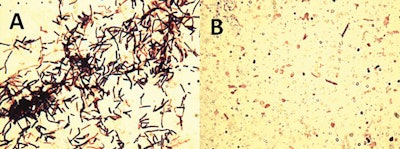
Lysis of Clostridium perfringens by a recombinant bacteriophage enzyme. Cells treated without phage lysin (A) or with lysin (B); note that all C. perfringens cells were completely digested with the addition of a recombinant bacteriophage-encoded lysin. (Bruce Seal, 2013)
Clostridium perfringens
With the removal of antibiotic growth promoters, there is an increase in cases of necrotic enteritis in poultry. The bacterium C. perfringens is responsible for these infections, alongside confounding factors. This Gram-positive spore-forming bacteria also causes a significant amount of foodborne disease in humans, as will as infections in other animal species. Scientists at the USDA’s Poultry Microbiological Research Unit identified lysins encoded by bacteriophages, which could be used to reduce levels of C. perfringens.
Screening material from poultry intestines, soil, sewage and wastewater from poultry processing plants, Seal and his colleagues searched for virulent bacteriophages from the Siphoviridae and Podoviridae double-stranded DNA virus families to lyse C. perfringens.
“Several bacteriophage genes that encoded N-acetylmuramoyl-L-al-anine amidases, lysozyme-endopeptideases and a zinc carboxypeptidase were identified,” Seal says. “Then we cloned the phage lysin genes and expressed them in Escherichia coli.”
The resulting amidases were tested for their ability to lyse the parental host strains, as well as other strains of C. perfringens. They did not, however, have any effect on other bacterial species — a measure of specificity and safety.
Animal models of infection and disease
C. perfringens can also cause intestinal health issues in piglets post-weaning and newborn calves, says David Donovan, formerly with the USDA ARS and adjunct professor at Morgan State University.
“We have demonstrated in vitro have that these lysins are effective against C. perfringens strains isolated from chickens, pigs and cattle,” Donovan says. “Next, we need to test the theory in live animals.”
Most of his work has been with Gram-positive pathogens of which Staphylococcus aureus is a major concern.
S. aureus is a pathogen that causes a broad spectrum of human and animal diseases and has adapted to antibiotic selective pressures resulting in a high prevalence of multidrug-resistant strains, e.g. Methicillin-resistant Staphylococcus aureus (MRSA).
“Lysins have been isolated from S. aureus bacteriophages, which have been effective in vitro with lytic assays,” Donovan says. “We have demonstrated their activity in the nasal passages, eye, muscle and bone, making them a potential alternative treatment for infections in humans.”
S. aureus is also a major cause of mastitis in dairy cattle, which can become chronic within the mammary gland.
“By fusing the lysins to protein transduction domains, we can show that they work intracellularly,” he says. “We hope to treat these intracellular infections and hopefully reduce numbers of cows culled.”
Commercial implications
Seal highlighted that, “in order for the concept to be utilized at a commercial level, production techniques need to be scaled up. For this, significant industry investment is required. However, much of the technology already exists and is used to produce feed enzymes and other additives.”
Donovan added that scientists first need to prove to the industry that this concept could be worthwhile.
“If you are going to feed the lysins to livestock, then you first have to ensure thermostability, so they aren’t destroyed in the feed pelletizing process,” Donovan says. “They also need to be tolerant to stomach acids, protease resistant and able to target just the gut pathogens without effecting important commensal strains.”
He and his colleagues have also engineered yeasts and plants to express the lysins.
“This avoids the need for a purification,” Donovan says. “The yeast or plant intracellular environments and natural chaperones help to protect the enzyme from heat and protease attack without the need for encapsulation or similar treatments.”
He also put forward the possibility of engineering yeasts used in ethanol production, such that DDGS co-products already contain gut pathogen targeting lysins.
“In this way, you are adding value to DDGS, producing the lysins during ethanol production and getting them directly into animal feed,” Donovan adds.
Safety considerations
Most phages do not directly display any risk to human health or the environment. However, there are mobile genetic elements that can interact with their bacterial host and, in some cases, genes are transferred between bacteria. Consequently, viral particles that possess certain properties may negatively affect human health by promoting bacterial virulence and pathogenicity or expressing toxins. Using phage lytic enzymes removes this risk.
Donovan explained that the parental phage and bacterial host have co-evolved for millennia.
“The lysin is a natural part of the phage life cycle, essential for cell lysis that allows the new bacteriophage to escape from the bacteria and infect other host cells,” he says. “Therefore, if the phage exists, then the bacteria cannot resist the lytic action of the phage lysin or the phage would have died out many generations ago.”
Because a large variety of phages exist for any given bacterium, the trend is to use a “cocktail” to ensure a variety of mechanisms, thus reducing the relatively high frequency of phage resistance exhibited by bacteria.
By using lysins, only the escape mechanism is exploited, and they can be used to kill many different strains of a particular bacterium.
Antibiotic alternatives
Seal concluded that lysins would work best as a preventative measure, added to the diet continuously as with other additives.
“For treatment, you would need to add a large amount in order to cover a severe infection,” he says. “You don’t have the benefit of them scaling up themselves, in line with the infection pressure, as you do with whole phage therapy.”
This is where the lytic enzyme concept comes into its own.
Once an effective lysin gene has be replicated, after the initial bacteriophage screening process, it can be used to reduce the population of all bacterial strains within the species. Scientists believe that bacteriophage gene products could eventually be used to target bacterial pathogens, such as C. perfringens.
This species-specific strategy could be used to control animal and human diseases without having deleterious effects on beneficial probiotic bacteria. Work to identify new endolysins as treatments for human and animal medicine is ongoing, along with endolysins that can target Gram-negative pathogens.
References available on request.