
There are many forms of mineral complexes and chelates available for use in animal nutrition, all of which are generically referred to as organic trace minerals (OTMs).
The chemistry of complexation or chelation has created a great deal of confusion in the animal feed industry, yet official definitions remain vague and unhelpful. As an example, some of the most recent changes to the classification of different zinc products authorized for use in the EU are summarized in the table, EU classification of organic zinc products, from which it is clear that official control definitions do little to differentiate between the various products on sale.
Typically speaking, OTMs are prepared by reacting inorganic mineral salts with different bonding groups including, organic acids, chemically synthesized amino acids, enzymatically-prepared mixtures of amino acids or small peptides produced from proteins and even polysaccharides. These different bonding groups bind the mineral and aim to ensure that the mineral becomes part of a biologically stable structure. Many different assertions are made as to the relative merits and suitabilities of different bonding groups in forming OTMs, with an even greater number of arguments existing in relation to their relative bioavailabilities.
The role of bond strength on OTM stability
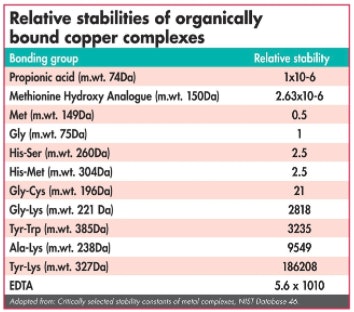
When trying to compare OTMs on the basis of “which is best under this set of conditions,” it can be useful to compare products in terms of the strength of the bond between the mineral and bonding group, using what are known as stability constants. In general, the higher this value is, the more stable the bond, and thus the greater the relative proportion of bound mineral will be. Obviously, there will be exceptions to this, but the table, Relative stabilities of organically bound copper complexes, compares how stably (or weakly) copper is bound by various bonding groups. As the type of bonding group changes, so too does the strength of bond between it and the copper group — some bond copper tightly, and some bond copper very weakly. The same holds true for all other minerals such as Zn, Mn and Fe.
The higher the stability of an organic trace mineral, the greater its bioavailability is likely to be.
What this indicates is that not only does the type of bonding group influence the stability of a given chelate, but for peptides, the sequence of amino acids will significantly influence how stably the mineral is bound. It also highlights that size is not as critical as claimed by some manufacturers. In essence, simple changes in the type of bonding group used to chelate the mineral will have a profound influence not only on the strength of bond between the mineral and bonding group, but more critically on the gastrointestinal stability of an OTM. Ultimately, the stability of an OTM is of paramount importance to its bioavailability.
Organic mineral stability dictates bioavailability
During transit through the gastrointestinal tract, in particular as the pH decreases or acidifies, all OTMs are subjected to conditions that can potentially result in the bound mineral being released as a charged free mineral ion. There are a number of negative consequences to this pH induced dissociation of OTMs. For instance, the charged, free mineral ion can react with negatively charged plant components, such as phytate, or worse still, can form a mineral-hydroxide upon reaching the small intestine. Both can lead to the precipitation of the mineral and a very significant reduction in bioavailability. Essentially, complexes or chelates with low stability or bond strength will not deliver the mineral to the sites of absorption in the intestine and reduce the effectiveness of the product to that of the corresponding inorganic salt. Chelation needs to maximize the pH-dependant stability of OTMs to increase mineral uptake in the intestine. In essence, the higher the stability of an OTM, the greater its bioavailability is likely to be.
Premix and feed antagonisms
The possibility for negative interactions occurring between individual components within premixes and feeds is high and often overlooked. Increasingly, the antagonistic effects of feed components have come under scrutiny.
Recent studies have focused on assessing these potential antagonisms. For instance, research has demonstrated that phytase inhibition is influenced by trace mineral type (see the figure, Dose-response curves representing the effect of Fe sources). This differential effect indicates that not all OTMs are created equal; moreover, they all differ in terms of their stabilities, releasing minerals in a pH-dependent fashion based on the pH in the local micro-environment. This instability results in some OTMs having a negative effect on enzyme activity.
In trace mineral premixes, oxidation-reduction reactions are the predominant cause of vitamin instability. The type of trace mineral will influence its reactivity and, more critically, the form that the trace mineral is presented in has an even more significant role to play in influencing vitamin stability. Studies examining Vitamin E stability in mineral premixes containing OTMs or inorganic sulphates demonstrate that inorganic minerals are detrimental to the stability of Vitamin E. Depending on the OTM source, however, the use of chelated minerals may not cause such a dramatic decrease.
Additional research assessing the effect of OTM source and concentration on the efficacy of antioxidants such as BHT has established that commonly used antioxidants can be compromised by the use of inorganic minerals (see figure, Effect of Iron source on antioxidant function). The data further indicates that in some cases, OTMs can also have a significant negative effect on antioxidant activity. Essentially, the use of weakly bonded minerals may result in a reduction in the efficacy of feed antioxidants such as BHT.
Importance of carefully choosing premix components
In general, OTMs have far less potential for reactivity compared to inorganic sources, however different forms of OTMs will react differently and cause greater or less inhibition of not only enzyme activity but also of vitamin stability and antioxidant function.
Overall, the available data demonstrates the importance of carefully choosing premix components and indicates that not all OTMs are created equal — some are more stable and less reactive than others. The consequences that these mineral induced antagonisms have for premix and feed formulation are tremendous and go some way toward explaining the variation noted in supplementation response between different organic trace minerals.
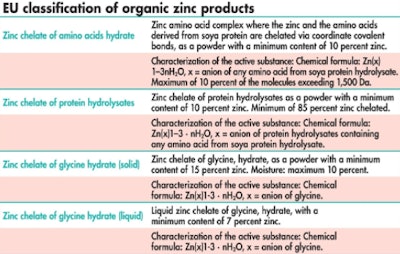
Official control definitions can lead to confusion when comparing organic trace minerals.
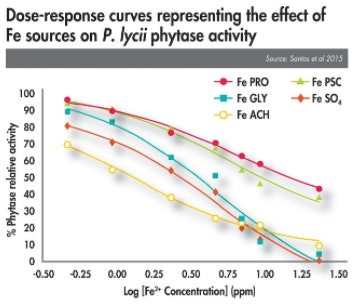
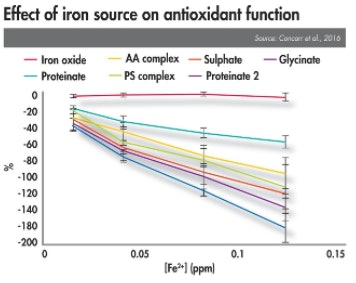
Not all organic trace minerals are equal — differences in stability influence how they interact with antioxidants.



















