Micro- and macrominerals are essential to the positive function of organisms.
In swine production, for example, iron and zinc are often supplied in piglets’ early life. To combat post-weaning diarrhea, which presents production challenges, zinc oxide (ZnO) is usually supplied at a pharmacological dose of 3,000 ppm to prevent this disease, reducing morbidity and mortality. Iron is also fed at high levels to combat anemia in piglets.
However, little is known about the possible negative effects of high iron levels or its bioavailability from the different iron sources supplied. The following article reviews available data on zinc, zinc oxide and the impact of excess iron in animal diets.
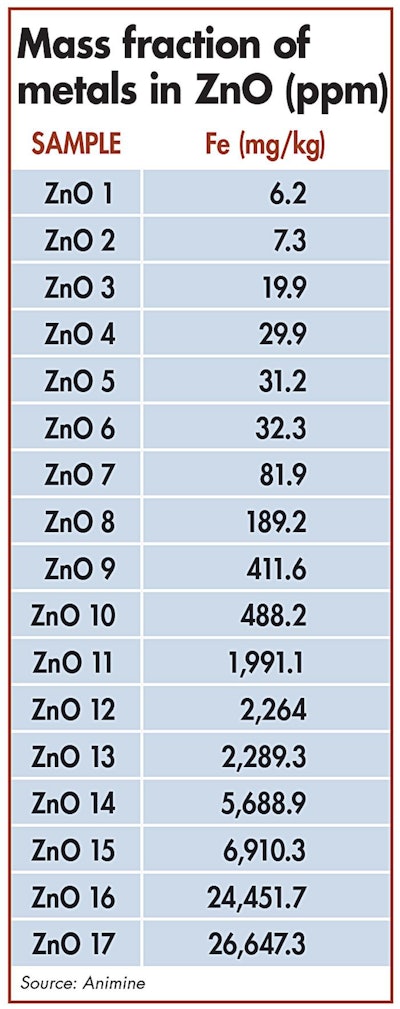
The source of the raw materials greatly influences variations in the presence of iron in zinc oxide sources.| ICP-OES
Study of zinc oxide sources
Depending on the manufacturing process and raw material sourcing, feed-grade zinc oxide (ZnO) materials can differ greatly from one to another. Macroscopically, different sources of ZnO vary in color, granulometry and density and they show diverse structural characteristics, such as particle size and shape, metal contamination, porosity, etc.
To better understand the properties of the different ZnO sources, one study analyzed more than 40 samples collected from feed industries worldwide. The samples were characterized and categorized according to their physicochemical properties, and their metal contamination was assessed according to the European Standard NF EN 15510 and analyzed by ICP-OES.
The presence of iron in ZnO sources can vary greatly according to the source of raw materials. Analyses have confirmed that some samples of ZnO have very high concentrations of iron, corresponding up to 2 percent of total zinc oxide.
For example, 2 percent iron content in zinc oxide sources fed to piglets at 3,000 ppm would correspond to 60 ppm of iron coming from zinc.
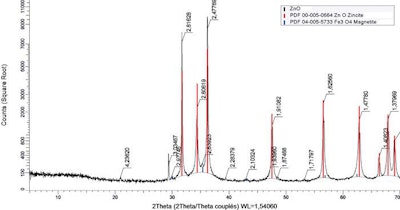
X-ray diffraction showing peaks corresponding to ZnO and iron oxide (magnetite – Fe3O4). Axis (x) represents the position recorded as the angle 2theta; (y) record the x-ray intensity as “counts.”
Iron bioavailability
To gain better understanding of the bioavailability of the iron present in ZnO, samples were characterized by X-ray diffraction to establish in which form iron is present.
Iron is mainly present in the form of iron oxide — magnetite (Fe3O4). EFSA considers iron oxide a source of iron that should not be used to meet animal requirements. Furthermore, EFSA does not state data regarding the biological fate of microparticles of iron oxide (Fe3O4).
Oxides normally are soluble at low pH; however, the dissolution rate depends on its physicochemical properties. If the iron present in zinc oxide cannot be dissolved in the stomach, it won’t be absorbed by the animals’ body and it will flow on the gastrointestinal tract where it can interact with other feed compounds, microbiota and intestinal epithelia. There is still little knowledge about the effect of excess iron in piglet feed; however, there is an increasing concern about this matter.
Effect of excess iron in animal feed
The following review of literature examines the health effects of excess iron in animal diets:
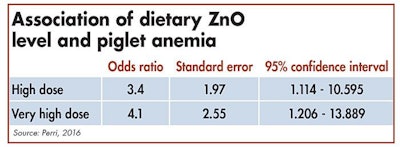
The odds of piglets being anemic is greater for pigs consuming high doses of dietary ZnO.
- Weaning piglets
Interactions between trace minerals are extensively reviewed in literature. In a recent study conducted in Canada, the effects of high doses of ZnO on iron status of weaning piglets were observed. Depending on the farm, piglets were fed with nutritional dosage (< 500 ppm), high dosage (2000-3000 ppm) or very high dosage (> 3000 ppm) of ZnO during the post-weaning period.
In conclusion, the odds of nursery pigs being anemic was three times greater for pigs consuming high doses of dietary zinc, compared with pigs consuming zinc at nutritional dosage, and four times greater for those fed starter diet containing very high dosage of zinc.
The mechanisms of this antagonism are not fully understood: the current hypotheses include a competition for the transporter DMT1 and an interference of zinc with the conversion of iron into ferritin.
Despite the high iron concentration in some ZnO sources analyzed, the use of these sources at pharmacological level can affect the iron status of piglets and consequently their performances.
- Negative side effects of iron
During the past decade, some studies conducted with mammals suggest that high levels of dietary iron can have negative effects on gut health. For example, a deleterious effect of 750 ppm of dietary iron on gut integrity was recorded in calves.
Similarly, a high dose of dietary iron (> 500 ppm) could increase the intestinal permeability of weaning piglets and alter the gut morphology, with villous atrophy. This effect on barrier function could be related to oxidative damages and lipid peroxidation in mucosa. These oxidative damages can harm not only the epithelia but also cause stress to local microbiota.
- Gut microbiota
Human studies have suggested that unabsorbed iron can stimulate growth and virulence of pathogens in the intestinal environment. Bacteria may solubilize iron oxide by lowering the external pH, reducing the iron to the more soluble ferrous forms or binding to siderophores.
Iron can bind to phytate and become unavailable to the host. However, studies in rats have shown that gut microbiota can degrade iron bound to phytate and use it for itself.
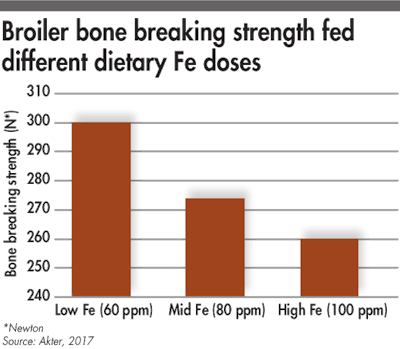
When dietary Fe increases, the tibia breaking strength decreases.
- Interaction with phytase
High dosages of iron could inhibit phytase efficacy. A recent study conducted in broilers showed a negative effect of high dietary iron (100 ppm) on nutrient utilization and on tibia breaking strength. Indeed, high iron levels in the gastrointestinal tract could lead to the formation of iron-phytate complex and reduce the phytate hydrolysis by phytase.
The literature implies that excess iron can comprise gut health and, therefore, animal performance. Hence, it can be suggested that iron present in ZnO is rather a contamination than a supplementation.
References available upon request.
Denise Cardoso and Agathe Romeo work in Animine's research and development development.