Trials demonstrate how livestock feed producers can do more with less
Around the globe, anticipated changes in livestock feed mineral inclusion regulations are shaking up historical approaches to mineral supplementation. This has led many to question not just how much mineral to add to various livestock rations, but also their source. When feeding more mineral is no longer an option, the key to navigating these regulatory hurdles is to consider technologies with a greater effect on the animal with less supplemental mineral.
Avoiding dissociation
One way to combat inclusion limits on trace minerals, such as copper, manganese and zinc, is to use sources that allow for greater mineral delivery to the site of absorption. To do this, a mineral source must be resilient to changes in pH, less prone to antagonism and provide enough ionized mineral to the site of absorption in the small intestine.
Low pH environments, like that in the stomach, can cause dissociation, the process where compounds separate into ions, resulting in a change to the molecular structure of the mineral source. This dissociation can result in the mineral source reacting with antagonists like phytate, calcium or phosphorus, lessening the mineral available for absorption in the small intestine.
The complexity of the mineral source’s bond can influence how well the metal is protected from dissociation in low pH environments. Some mineral sources with undefined structures are prone to rapidly dissociate early in the gastrointestinal tract, leaving the mineral exposed to antagonists.
Choosing a mineral source
More defined mineral sources, like metal methionine hydroxy analogue chelates (MMHAC), have a defined structure consisting of chelated bonds that protect the mineral across a wide pH range.
A benefit of maintaining molecular structure through resisting changes in pH is that the mineral is also less susceptible to antagonists. Antagonists are dietary factors that bind to the mineral as it and digesta flow through the gastrointestinal tract, rendering the minerals unavailable to the animal. MMHAC provides protection to the mineral against antagonists because of its unique chelated structure featuring two molecules of methionine hydroxy analogue (2-hydroxy 4-(methylthio) butanoic acid or HMTBa) for every molecule of mineral.
The metal is protected by four coordinate covalent bonds, resulting in a neutral molecule. Mineral sources that do not possess a neutral charge look to bind with other compounds, form a neutral charge and increase their stability. However, when minerals bind with antagonists like phytate, calcium or phosphorus, the bond between them is so strong that very little in the digestive tract can liberate the mineral from these complexes. This leads to decreases in mineral utilization by the animal and ultimately limited productivity.
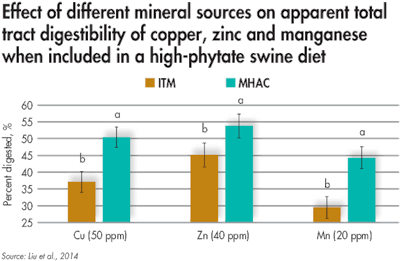
Pigs fed a high-phytate diet experienced more mineral absorption from metal methionine hydroxy analogue chelates (MMHAC) compared with sulfates. (Novus International Inc.)
In a study where a ration high in phytate was fed, apparent total tract digestibility of copper, zinc and manganese were increased when these minerals were supplied as MMHAC (Cu-MHAC, Zn-MHAC and Mn-MHAC, respectively) compared with sulfates. This data demonstrates that despite the high levels of phytate, more mineral from MMHAC was able to be absorbed and utilized by the animal, resulting in greater apparent digestibility.
Trace mineral absorption
The goal for all trace minerals supplemented in livestock rations is removal from the digesta and entrance into the blood stream, a process known as absorption.
To be absorbed, trace minerals must be soluble in the digestive fluid. Trace minerals are absorbed throughout the small intestine by specific transporters located on the surface of enterocytes, cells that line the small intestine and specialize in absorption. This allows transporters, specialized proteins located in the enterocyte’s cell membrane, to bind minerals moving them from the digesta and into the enterocytes.
For zinc, the zinc importer (ZIP) and zinc transporter (ZnT) families actively bind and transport the mineral through the membrane of the enterocytes of the small intestine. The predominant zinc transporter into the enterocytes is Zip4. Due to the high affinity of these transporters for zinc, the mineral is efficiently pulled from the passing digesta and enters the enterocyte where it waits until it passes into the bloodstream.
Once trace minerals have entered the bloodstream, they can be shuttled to different tissues to meet the mineral needs of the animal. This is a tightly regulated and specific process as zinc can be toxic when it accumulates and reaches a high level, so the body has developed several mechanisms, including chaperon proteins, to safeguard against zinc freely entering enterocytes or the bloodstream.
Zinc, enterocytes and the effects on metallothionein
When zinc is adequately supplied, expression of metallothionein, a chaperon protein, increases to sequester zinc and, to a lesser degree, copper. As a chaperone protein, metallothionein transports zinc through the enterocyte so that it can enter the bloodstream when needed. This prevents zinc from freely entering the bloodstream where it can pose a risk for toxicity. Therefore, when zinc concentrations increase in the enterocyte, metallothionein expression increases to better handle incoming zinc.
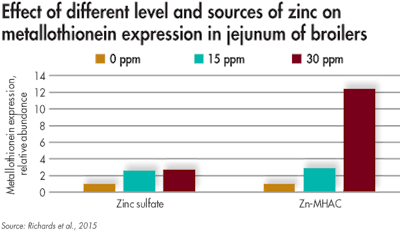
A study of the effects zinc sources and levels have on metallothionein expression in the jejunum of broilers revealed that Zn-MHAC supplementation of 30 ppm resulted in more mineral absorption than with other sources. (Novus International Inc.)
To better illustrate this phenomenon, a study was conducted to determine how the effect of zinc supplementation level and source affected metallothionein expression in the jejunum of broilers.
When the level of zinc increased from 0 to 15 and 30 ppm, metallothionein expression increased; however, when zinc methionine hydroxy analogue chelate (Zn-MHAC) was supplemented at 30 ppm, the response was even greater. This suggests that, due to the chelation technology associated with Zn-MHAC, more mineral is being absorbed by the enterocytes. With the increase in zinc absorption, more zinc can reach areas in the body where it is needed, optimizing livestock productivity.
Furthermore, this data highlights that the response in metallothionein from 15 ppm Zn-MHAC is the same as that of 30 ppm zinc sulfate, suggesting that the animal can do more with the zinc coming from Zn-MHAC even though less is supplemented.
Zinc availability
Increasing chaperon proteins in enterocytes is a good measure of greater zinc availability, but it falls short of proving that the zinc supplemented is reaching the tissues in the animal where it needs the mineral the most.
In human research, a method to feed zinc with different numbers of neutrons, known as stable isotopes of zinc, and to detect the appearance of the stable isotopes in samples has historically been used.
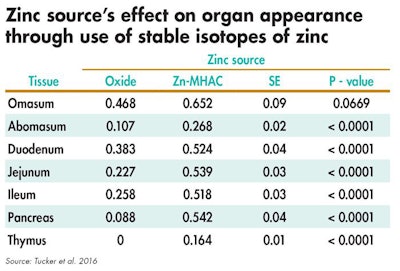
Steers were fed several zinc sources to determine how zinc isotopes deposited in different tissues. The study found gastrointestinal tract tissues had greater zinc uptake from Zn-MHAC compared with zinc oxide. (Novus International Inc.)
In a recent study, stable isotopes of zinc were used to make zinc oxide and Zn-MHAC. These zinc sources were then fed to steers in order to determine to what extent and to which tissues the zinc isotopes were deposited. Tissues in the gastrointestinal tract had greater zinc uptake from Zn-MHAC compared with zinc oxide. This could represent the gastrointestinal tract’s involvement with zinc absorption, but also could signify its large metabolic need for zinc because of its role as the body’s first line of defense when it comes to immune function and the high cellular turnover.
Second to the gastrointestinal tract, the thymus and pancreas had greater zinc from Zn-MHAC compared with zinc oxide. Thymus and pancreas are key endocrine glands that function to maintain the immune system and glucose metabolism, respectively. In their roles, both organs utilize a large amount of zinc for the immune cell and enzymes they generate. By detecting more zinc in key tissues with the use of stable isotopes, this demonstrates that, regardless of the challenges Zn-MHAC faces in the gut, more mineral is delivered to the animal.
Regulatory guidance concerning application of trace minerals will surely continue to evolve, but the need to supplement mineral sources that can survive the challenges in the intestine will remain constant. Through providing a mineral source that can limit its dissociation in the acidic environment of the stomach, increase the expression of metallothionein by having more mineral reach the site of absorption and reach tissues with high need for zinc, livestock producers can expect similar or better outcomes for their animals.
Effectively, this means more can be done with less supplemental mineral when a proven source is considered.