Key points:
- Methionine hydroxy analogue – free acid (MHA-FA) contains 23% oligomers that are not readily absorbed in the intestine and are largely recovered in excreta.
- The absorbability of MHA-FA is dependent upon low pH meaning it is only effectively absorbed in the upper GI tract.
- MHA-FA is not effective at prohibiting bacterial growth due to MHA-FA’s large molecular size which inhibits its ability to disrupt bacterial cell functions with practical feeding applications.
Comparing bio-efficacies:
Over the last couple of decades, resources have been utilized to determine the comparative nutritional efficacy of methionine hydroxy analogue free acid (MHA-FA) to its dry counterpart, DL-methionine (DL-Met). During this time, a multitude of research studies have evaluated sources of methionine and effectively raised DL-Met to be the “gold standard” source for its 100% availability. With 12% of its mass being water, MHA-FA is approximately 88% analogue which is divided into 65% monomers and 23% oligomers. Mitchell and Lemme (2008) observed that intestinal absorbability of MHA-FA is reduced compared to DL-Met. Applying intestinal loop perfusion technique, Mitchell and Lemme (2008) reported 17-29% lower absorption of MHA-FA compared to DL-Met under various temperature regimes and intestinal conditions free of microbes. Moreover, they reported that absorption of MHA-oligomers was only 1-2% of total MHA-FA absorption.
Agreeing with Mitchell and Lemme, research by Van Weerden et al., (1992) demonstrated poor absorbability of MHA-FA and especially MHA-oligomers as the relative bio-availability of MHA-FA and MHA-oligomers determined in two experiments was 76% (67.5% on product basis) and 56% on equimolar basis relative to DL-Met in broilers. The European Food Safety Authority (EFSA) supports this argument with a recommended bio-efficacy of MHA-FA at 75% on an equimolar basis (66% product basis) (EFSA, 2018).
Interestingly, Drew et al., (2004) found that microbial degradation is responsible for a large percentage of the low bio-availability of MHA-FA in the small intestine. The neutral pH found in the small intestine provides for microbial degradation as the absorbability of MHA-FA is reduced as pH increases. Differences between methionine sources are also partly due to the methods of how each source is absorbed into the intestinal cells. DL-Met is readily absorbed via sodium (Na) dependent transporters, while MHA-FA is absorbed through proton dependent monocarboxylate transporters (MCT) coupled to the activity of the Na+-H+ exchanger (NHE3) (Martin-Venegas et al. 2007) which is also where fatty acids are regulated. MHA-FA’s dependency upon H+ transporters in the lower GIT for absorption combined with a reduced absorption of MHA-FA oligomers effectively support a bio-efficacy that is much lower than 88%.
Animal producers often choose to utilize organic acids as they are thought to reduce pathogenic bacteria of concern to food safety while having the flexibility to easily apply in water applications or in feed. In animals, organic acids are lipophilic at low pH (Knight and Dibner, 1984), meaning that once ingested it enters the bacterial cell and then dissociates to form a proton and anion that reduces the pH intracellularly. The reduction of pH intracellularly forces the cell to utilize its proton pump (requiring ATP) to correct the pH, resulting in depletion of ATP and the death of the cell. However, as the organic acid moves through the GIT, it changes from its undissociated form in the foregut to its dissociated form in the small intestines correlating with increasing environmental pH.
It is sometimes claimed that MHA-FA shows acidifying effects and would be a methionine source and an acidifier at the same time. With a pKa value of 3.5, MHA-FA is stronger compared to some commonly used organic acids (propionic, butyric, sorbic, acetic and formic). Indeed, MHA-FA is an organic acid with both low pH and pKa values. Thus, MHA-FA should have majority of its interaction in the upper, more acidic GIT. As MHA-FA transitions into the small intestines, will have increasingly reduced absorbability and a reduced ability to donate H+ ions. The “strength” of an acid is based upon its ability to dissociate and donate a proton (often as a H+ ion). Because of this, it is thought that utilizing MHA-FA can inhibit salmonella in animal feed (pH ~7). However, molecular weight must also be considered when determining strength as it is corelated to the number of molecules in a solution. An organic acid with a low molecular weight would have an elevated ability to donate H+ ions as there would be more molecules of the acid compared to an organic acid with a higher molecular weight.
It was demonstrated by Strauss and Hayler (2001) that increasing molecular weight (g/mol) utilizing 3 different organic acids (Formic, 46 g/mol, pKa- 3.75; Propionic, 74 g/mol, pKa- 4.88; and Lactic acid, 90 g/mol, pKa- 3.83) led to an increased minimum inhibition concentration of different bacteria regardless of pKa. This interaction is likely due to the increased number of small molecules relative to large molecules able to have an increased number of donatable protons. Therefore, work by Strauss and Hayler (2001) suggests a correlation between molecular weight and bacterial inhibition.
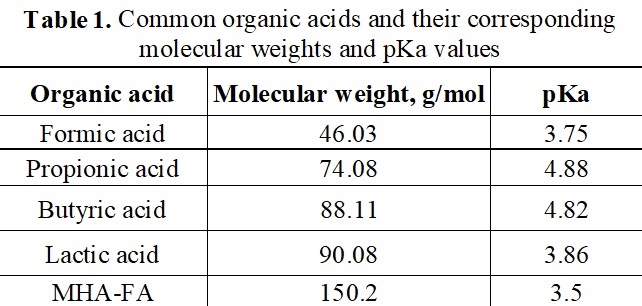
Image courtesy of Evonik
The molecular weight of MHA-FA is very large (150.2 g/mol) compared to common organic acids (Table 1). Although the pKa of MHA-FA is low, its high molecular weight could weaken its anti-bacterial effect as it will not be able to donate as many protons. A study was conducted by Abbott Analytical in Helsinki, Finland (Locatelli et al., 2006) to evaluate the inhibitory effect of formic acid (FA; 85% concentration) and a blend of FA and its ammonium and sodium formate salt (AS; 40% FA), MHA-FA (88% purity), DL-methionine (DL-Met; 99% purity) and combinations of FA and methionine sources on the presence of Salmonella typhimurium in feed. Results (Table 2) (Locatelli et al., 2006) indicated no differences in Salmonella recovery between DLM and MHA-FA at any time point; however, the use of FA and AS did lead to significant reductions of Salmonella. Additionally, no added benefits were observed when adding DLM or MHA-FA to either FA or AS.
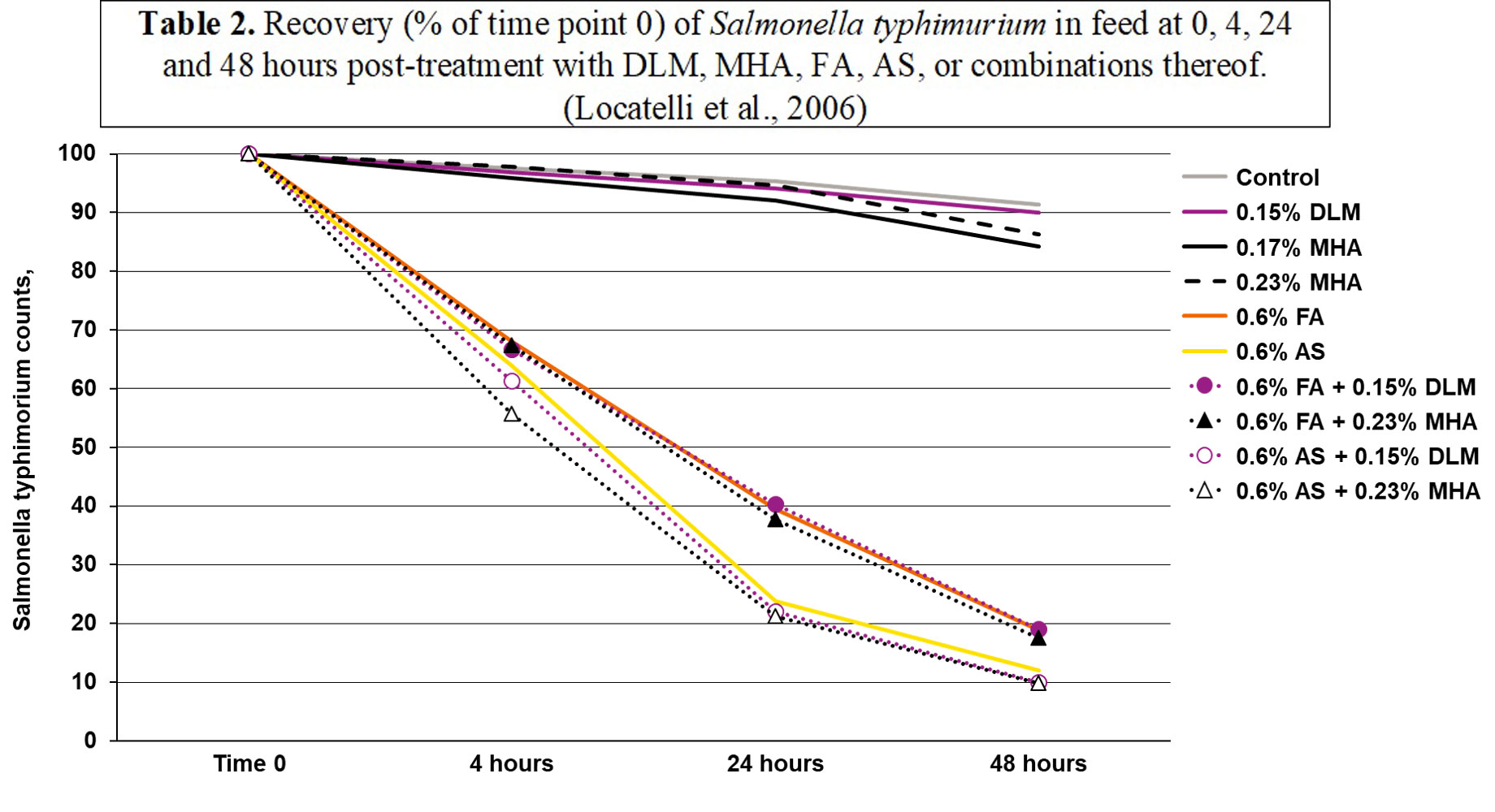
Image courtesy of Evonik
A further study was conducted in which digesta of pigs and broilers fed diets without any methionine source for 10 days was obtained for an in-vitro assay (Evonik, unpublished). The samples were either spiked with equimolar dosages of DL-Met, MHA-FA or remained untreated before they were incubated in bio-reactors with a specific pH of 5.5 (representing the proximal intestine) and pH 6.5 (representing the distal intestine) at 38°C under anoxic conditions. Samples were taken at 0, 1, 2 and 4 hours of incubation. These incubations appear to be a good simulation of digestion dynamics as the digesta of pigs remains in the small intestine for about 2-6 hours while avian species have retention times in the small intestine of about 3-4h. Results (Table 3) indicate that incubation time slightly affected the pH and counts of total anaerobe microbes, but there was no differentiation between the three treatments at the proximal or distal intestine.
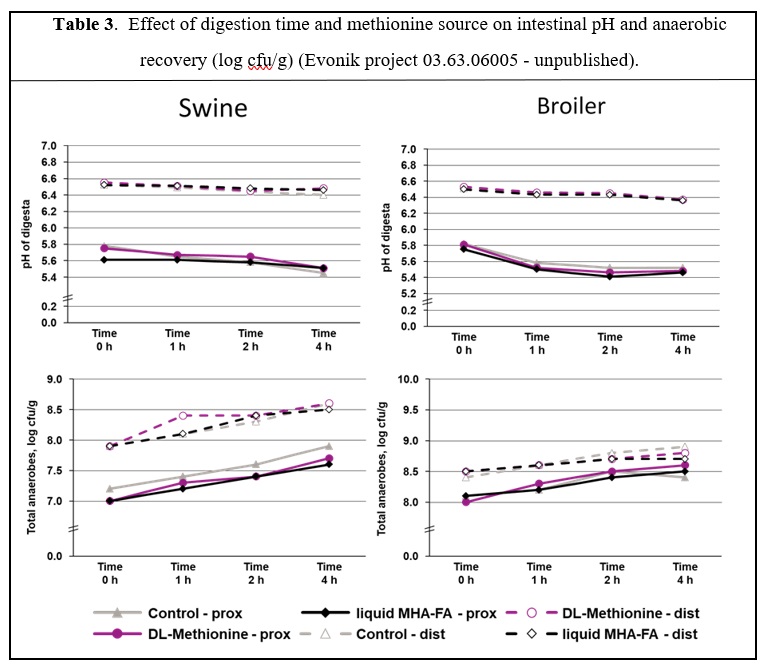
Image courtesy of Evonik
The bio-efficacy of MHA-FA is reduced by its existence in both monomeric and oligomeric forms which have differing digestibility. In addition, intestinal microbes degrade a certain portion of supplemental MHA-FA. Finally, MHA-FA’s bio-efficacy is also reduced by its nature as an organic acid as its inability to donate H+ ions in a neutral pH makes it increasingly difficult for absorption. Therefore, MHA-FA’s dependence upon low pH environments for absorption makes it an inferior choice as a methionine source and should also not be considered as a multi-role ingredient as it cannot effectively impede bacterial growth.
Dr. Kyle Smith, Dr. James Wen, Dr. Maria Mendoza, Evonik Corporation, Kennesaw, GA, USA
